Mysimba®

Mysimba is a medicine used along with diet and exercise to help manage weight in adults:
• who are obese (have a body-mass index – BMI – of 30 kg/m2 or more);
• who are overweight (have a BMI between 27 kg/m2 and 30 kg/m2) and have weight-related complications such as diabetes, abnormally high levels of fat in the blood, or high blood pressure.
(BMI is a measurement that indicates body weight relative to height.)
The two active substances, naltrexone and bupropion, act on the parts of the brain that control food intake and energy balance, as well as reducing the effect of the part of the brain that controls the pleasure associated with eating food. When given together, their actions reduce appetite and the amount that patients eat, and increase energy expenditure, helping them to stick to a calorie-controlled diet and to reduce their body weight.
The effect of Mysimba has been proven in four randomised, controlled trials. Patients using Mysimba averagely loose 4-5 times as much weight as a patient on placebo medication.1 50% of patients respond to Mysimba, with a mean weight loss of 11,5 %, one year after starting the medication.2 To be qualified as a responder, the patient needs to loose 5% of initial body weight within 16 weeks of the start of medication.
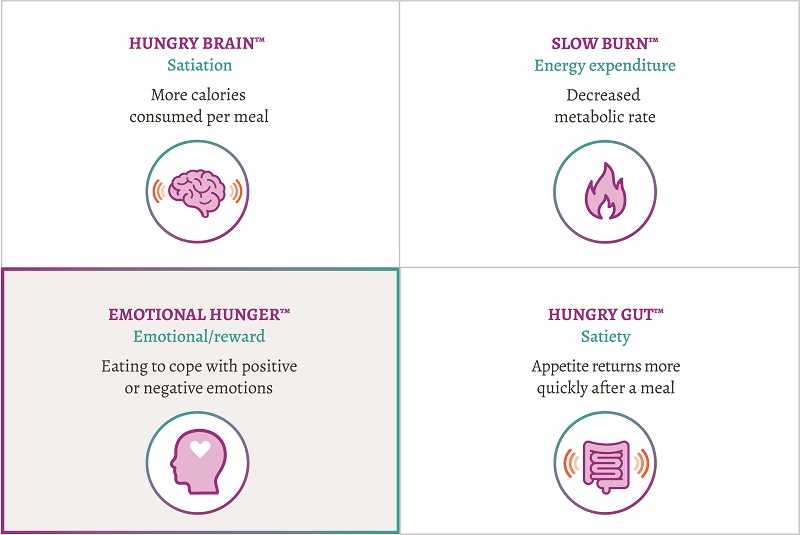
Emotional eating — a challenge for weight-loss success
When it comes to creating a weight-loss plan for your patients, one size doesn’t fit all.3 Different causes of obesity create unique challenges for weight-loss success. The Acosta et al observational study found that an individualized treatment approach based on 4 phenotypes was associated with significantly greater weight loss after 12 months compared with the non–phenotype-guided groups. For some patients, this means addressing their cravings that can be associated with emotional eating.3 Mysimba is designed to reduce hunger and control cravings so your patients who are overweight or struggling with obesity can lose weight and keep it off.1,4
Mysimba is currently reimbursed in Norway and Finland. For more information about reimbursement criterias, please see:
Norway Reimbursement Criterias
Finland Reimbursement Criterias
References:
- Greenway et al (2010) Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010 Aug 21;376(9741):595-605.
- Fujioka et al (2016). The relationship between early weight loss and weight loss at 1 year with naltrexone ER/bupropion ER combination therapy. Int J Obes (Lond). 2016 Sep;40(9):1369-75
- Acosta A, Camilleri M, Dayyeh BA, et al. Selection of antiobesity medications based on phenotypes enhances weight loss: a pragmatic trial in an obesity clinic. Obesity (Silver Spring). 2021;29(4):662-671. doi:10.1002/oby.23120
- European Medicines Agency: https://www.ema.europa.eu/en/medicines/human/EPAR/mysimba